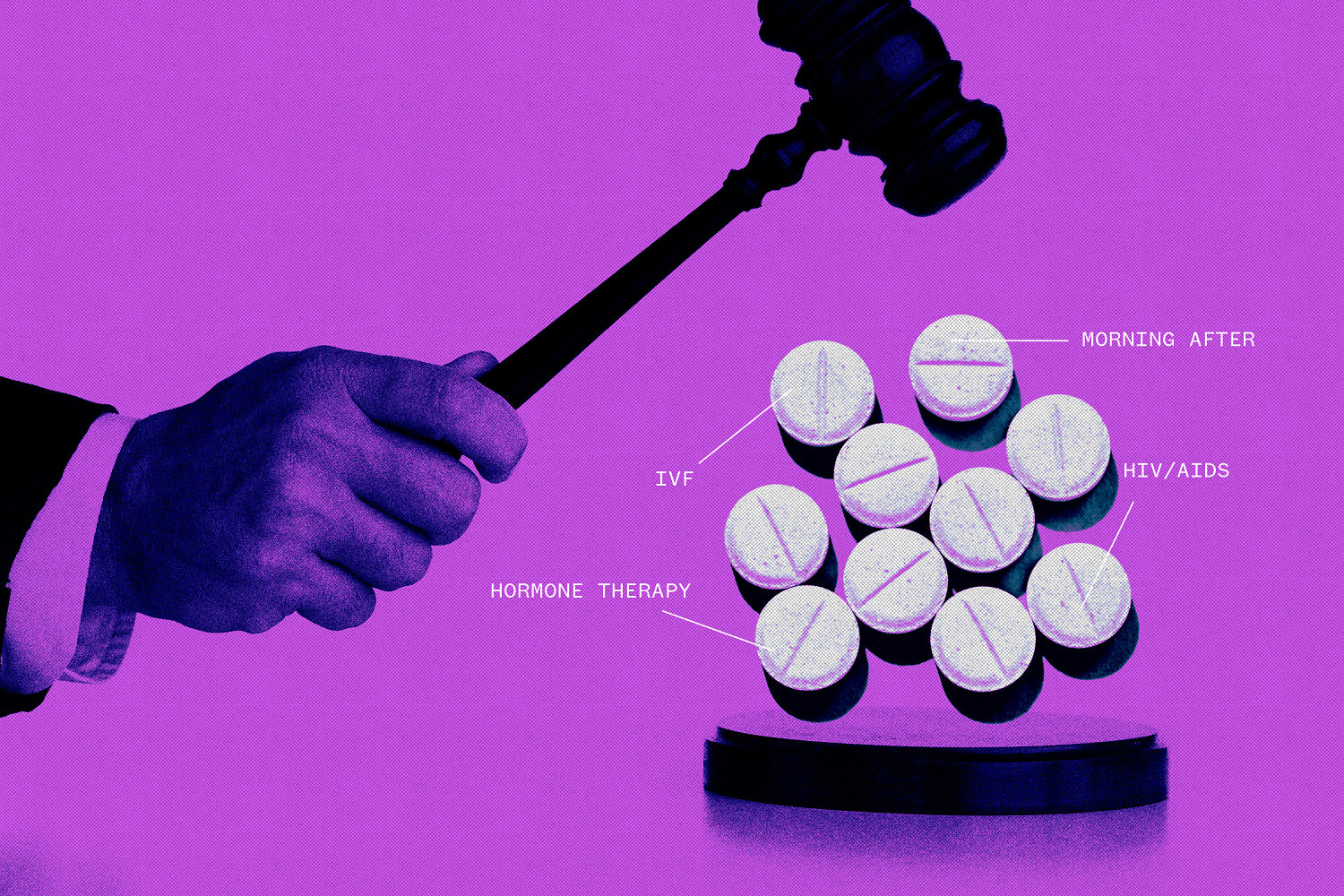
WASHINGTON — Vaccines, birth control pills, hormone therapies and fertility drugs would be subject to new litigation if the Supreme Court endorses a challenge to abortion pill mifepristone, pharmaceutical industry experts warn.
When the court on Tuesday weighs whether to roll back Food and Drug Administration findings that made mifepristone more readily available, it is not just access to that particular drug, used for the majority of abortions nationwide, that is on the line.
The pharmaceutical industry has raised the alarm, telling both the justices in court filings and anyone else who will listen that giving individual federal judges the power to cast aside the agency’s scientific health and safety findings would cause chaos within the sector.
It would likely lead to litigation over other drugs, both current and those yet to be approved, on which people have strong feelings.
If the anti-abortion groups win, “anyone with an ideological disagreement, coupled with a scientifically untrained judge, could challenge the FDA’s authority,” said Amanda Banks, a physician and entrepreneur who signed a brief along with dozens of other pharmaceutical executives and companies backing the FDA.
The main pharmaceutical industry group, PhRMA, also filed a brief in support of the government.
Some activists have long railed against certain vaccines, claiming without evidence that they can cause autism. During the Covid-19 pandemic, there were largely unfounded concerns raised by vaccine skeptics that the vaccines were not safe.
Anti-abortion activists, some of whom oppose all contraceptives, have long opposed the morning-after pill, viewing it as akin to abortion despite evidence suggesting otherwise.
LGBTQ activists have called on the FDA to specifically approve the use of hormone therapies for gender-affirming treatments. Groups that oppose gender-affirming care for transgender youth have asked the FDA to prevent puberty blockers from being prescribed to minors.
Other medications that could be subject to challenge include those developed using embryonic stem cells, drugs that treat HIV/AIDS and fertility drugs used for in vitro fertilization, industry experts and others who back the FDA say.
For business leaders, there’s also a concern a ruling against the government could stifle innovation by deterring investors in an industry that relies on billions of dollars in upfront research and development in order to bring drugs to market.
“My biggest concern is the precedent it sets … could have a chilling effect on investors coming into our business and investing in our innovative companies,” said Paul Hastings, an industry veteran who is the CEO of Nkarta Therapeutics and signed on to the same brief as Banks.
He and others stressed that the FDA is considered the “gold standard” of drug regulation worldwide. For investors, FDA approval is the final part of a rigorous and expensive process. Only 1 in 10 drugs under development ever end up being marketed, Hastings said.
Investors might look for safer bets if FDA approval merely leads to constant litigation, he added.
In court papers, the FDA itself said that no court has ever restricted access to an approved drug “by second-guessing FDA’s expert judgment about the conditions required to ensure that drug’s safe use.”
When the agency in 2016 began the process of lifting restrictions on the drug, its actions were “supported by an exhausting review of a record including dozens of scientific studies and decades of safe use of mifepristone by millions of women,” the brief said.
The challengers, doctors and other medical professionals who oppose abortion argue that FDA failed to sufficiently take into account safety concerns when the restrictions on mifepristone were lifted.
Erin Hawley, a lawyer with conservative Christian group Alliance Defending Freedom, who is arguing the case for the plaintiffs, rejected the notion that the case could have such far-reaching effects, calling it a “red herring.”
She said in an interview that the FDA has not identified any other drug approval that would be threatened.
“The reason being is that what FDA did in deciding to remove long-standing protections for women who choose to take mifepristone is quite extraordinary,” she added.
ADF has no plans to challenge any other drug approvals, a spokeswoman said.
The Supreme Court has a 6-3 conservative majority hostile to abortion rights, as illustrated most starkly by the 2022 ruling that overturned abortion rights landmark Roe v. Wade.
The legal question in Tuesday’s case does not focus on abortion itself but in part on whether the FDA followed the correct processes in easing restrictions on mifepristone.
The plaintiffs sued in a federal court in Texas where the case was guaranteed to be assigned to Matthew Kacsmaryk, a conservative judge appointed by President Donald Trump.
Kacsmaryk in April of last year issued a sweeping ruling that invalidated the FDA’s approval of the drug decades ago.
That ruling was put on hold by the Supreme Court and has subsequently been narrowed by the 5th U.S. Circuit Court of Appeals.
The original approval of mifepristone in 2000 is not at issue before the justices. The case focuses on FDA actions from 2016 onward that made it easier to access the pill, including the initial 2021 decision that made it available by mail, which was finalized last year.
The justices will also probe 2016 decisions to extend the window in which mifepristone could be used to terminate pregnancies from seven weeks’ gestation to 10 weeks and reduce the number of in-person visits for patients from three to one. In another 2016 move, the FDA altered the dosing regimen, finding that a lower dose of mifepristone was sufficient.
The FDA-approved regimen for a medication abortion involves two drugs: mifepristone, which blocks the hormone progesterone, and misoprostol, which induces contractions.
That the justices put Kascmaryk’s ruling on hold could be a sign that a majority is inclined to reject the challenge. Only two of the nine justices, conservatives Clarence Thomas and Samuel Alito, objected to that decision.
It is possible the court could resolve the case without delving into the knotty legal issues around the FDA’s approval process. The government has argued strenuously that the doctors and others who filed the lawsuit do not have legal standing because they cannot show any injury that can be traced to the FDA’s decisions.
The doctors themselves do not prescribe mifepristone, but they suggest they are injured because they could be required to treat patients who have taken the pill and have serious side effects. As they oppose abortion, any actions they are forced to take to help a woman complete the process would make them complicit, the plaintiffs argued in court papers.
The FDA’s lawyers wrote in the government’s brief that the plaintiffs can at best point at a “hypothetical scenario,” which is not enough to establish standing.
The challengers, the brief stated, “cannot identify even a single case where any of their members has been forced to provide such care.”